
PhD fellowship available: We are looking for good students with an interest in synthesis, host-guest chemistry and computational chemistry.
The fellowship is related to the "QC4ALL" and "ConChi" projects from the Ministerio de Ciencia, Innovación e Universidades (MICIU) and Agencia Estatal de Investigación. The official recruitment will be done through the formal procedures at the Universitat de Girona (following the guidelines of the Agencia Estatal de Investigación (AEI)), but we will be pre-screening candidates until September 12, 2024.

There is an overwhelming amount of data that indicate a direct relationship between catalytic activity and enzymes and/or the presence of transition metals in biomimetic complexes. The exact relationship between changes in the structure (through either modifications of the ligand, or mutations in the enzyme) and/or electronic structure (e.g. spin or oxidation state of the metal) with changes in activity are poorly understood. Therefore, modifications of activity and spectroscopy are governed by these changes in (electronic) structure. Through the application of computational chemistry techniques we can understand, and predict, the origin of these changes in reactivity.
Since the interactions between electrons and nuclei determine everything, we can study nanomaterials accurately, and through density functionals also efficiently. The proposed project is based firmly on quantum-chemistry in all its different guises, ranging from high-level wavefunction methods for reference data in benchmarking, through new density functionals that will be developed within the project. These improvements will be applied for investigating the mechanistic basis of metal- and enzyme-based catalysis, and validate the accuracy of computational spectroscopy techniques. Especially the time-resolved (resonance) Raman spectroscopy, which shows a richness of information compared to other techniques, shows great promise through a combined experimental-computational approach.
We use the application in spectroscopy of computational chemistry techniques which can be done accurately enough to be able to compare to experimentally observed spectroscopy. Hence, it can be used to predict modifications for the rational design of more effective and potent catalysts, with corresponding changes in spectroscopy. In summary, the current project aims to understand the mechanistic basis of metal- or enzyme-based catalysis and the molecular structure of nanomaterials.
The emergence of supramolecular chemistry in the 70’s was driven by a keen interest of synthetic chemists in mimicking the molecular recognition phenomena occurring in nature, with the ultimate aim of replicating the interesting and complex functions of biological hosts. Chirality is an intrinsic property of biological matter that underpins some of these functions. In proteinogenic receptors, chiral information is transferred from the peptide backbone to the bound substrates through complex molecular recognition processes that have been seldom replicated in synthetic hosts. While it is relatively easy to build up hosts with chiral scaffolds, efficient relay of the chiral information into the binding site is less obvious and remains the main challenge in this area. We hypothesize that this is due the lack of receptors displaying induced fit and conformational selection behavior. These interrelated concepts are an expression of the plasticity of biological receptors that is central to their outstanding capabilities.
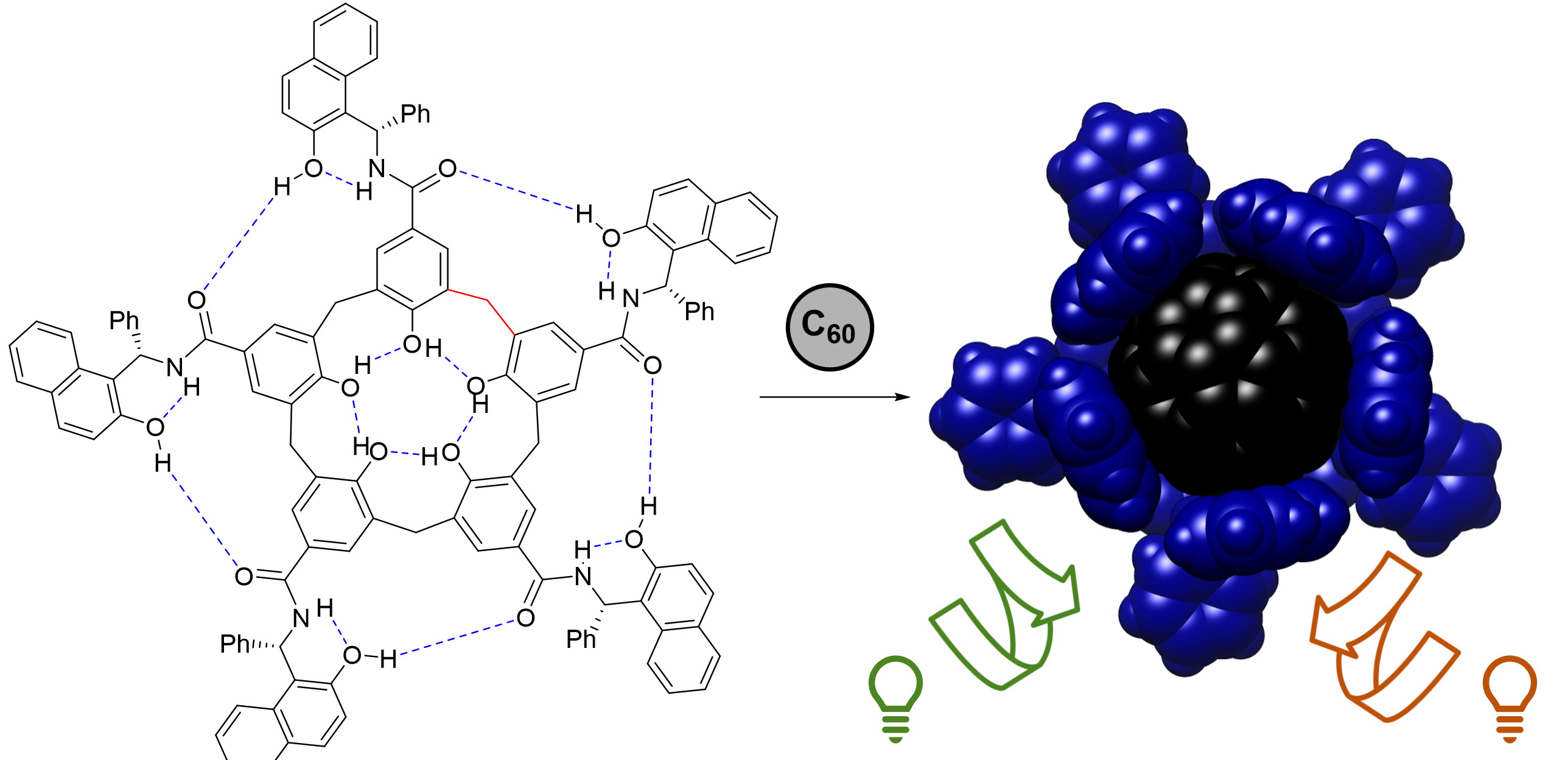
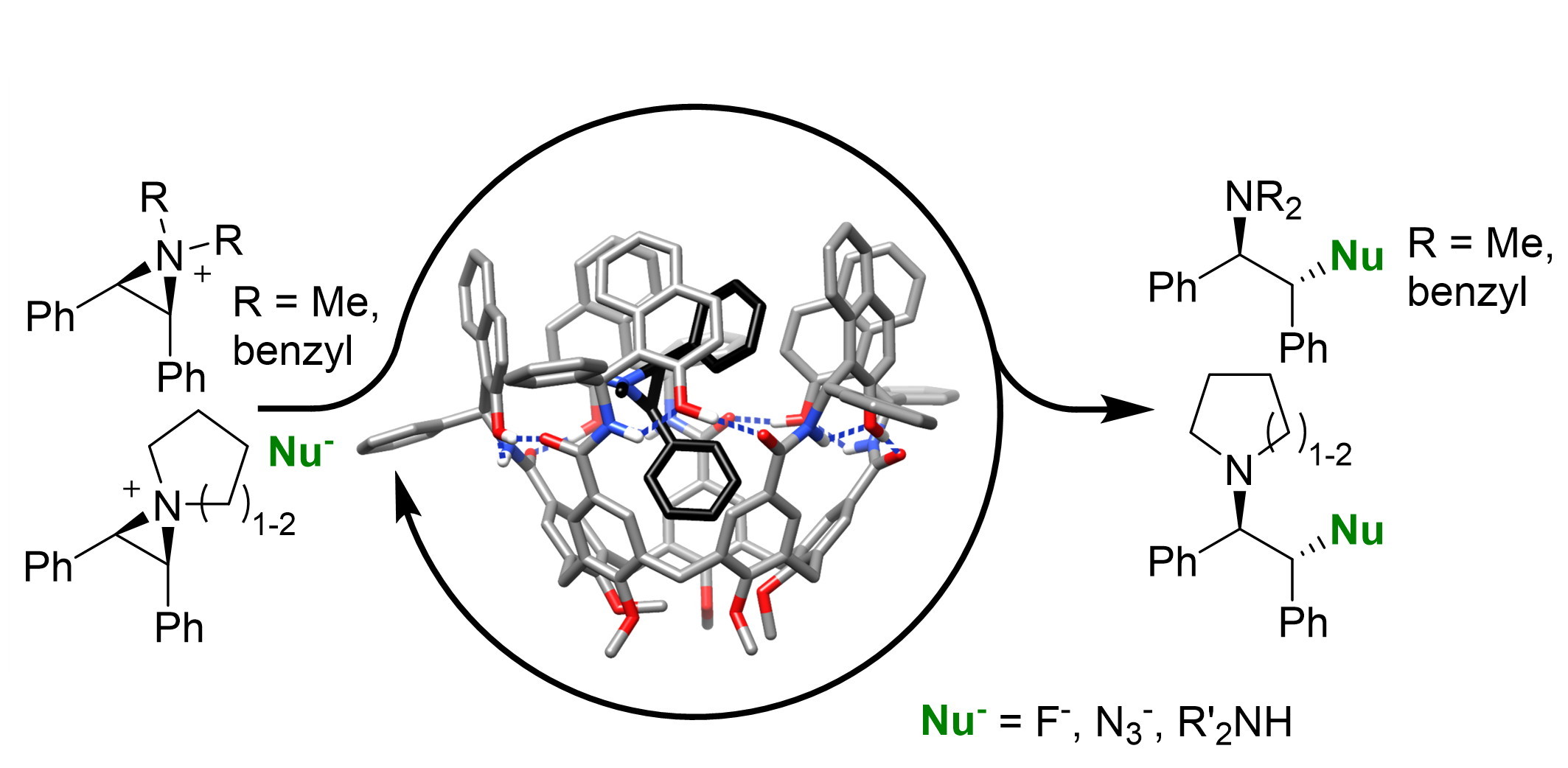
Our group has recently developed a family of self-folding cavitand receptors displaying genuine induced fit behavior. These hosts are based on a calix[5]arene (C5A) scaffold and are stabilized in closed yet flexible conformers, thanks to a bioinspired hydrogen bond network and reduced covalent bridging. A chiral C5A host (H1) has been shown to provide outstanding results in enantioselective molecular recognition. Herein, we will develop a new family of flexible chiral receptors with different sizes, shapes and electronic properties based on the H1 prototype, and we will exploit them in two novel applications.
We will develop proof-of-concept examples of enantioselective catalysis. We will study ring-opening reactions of aziridinium cations mimicking the privileged guests previously identified, providing enantioenriched chiral amines of interest. Alternatively, we will develop water soluble receptors to enhance binding of substrates through the hydrophobic effect, and we will study epoxide hydrolysis reactions in aqueous media. The proposed receptors depart significantly from the state-of-the-art in supramolecular enantioselective catalysis, which is dominated by highly symmetrical structures with poor induced fit behavior and not amenable to diversification. The new host proposed will be accessed by a late-stage diversification approach leading to both enantiomers of the desired hosts without the requirement of tedious chiral HPLC purification.
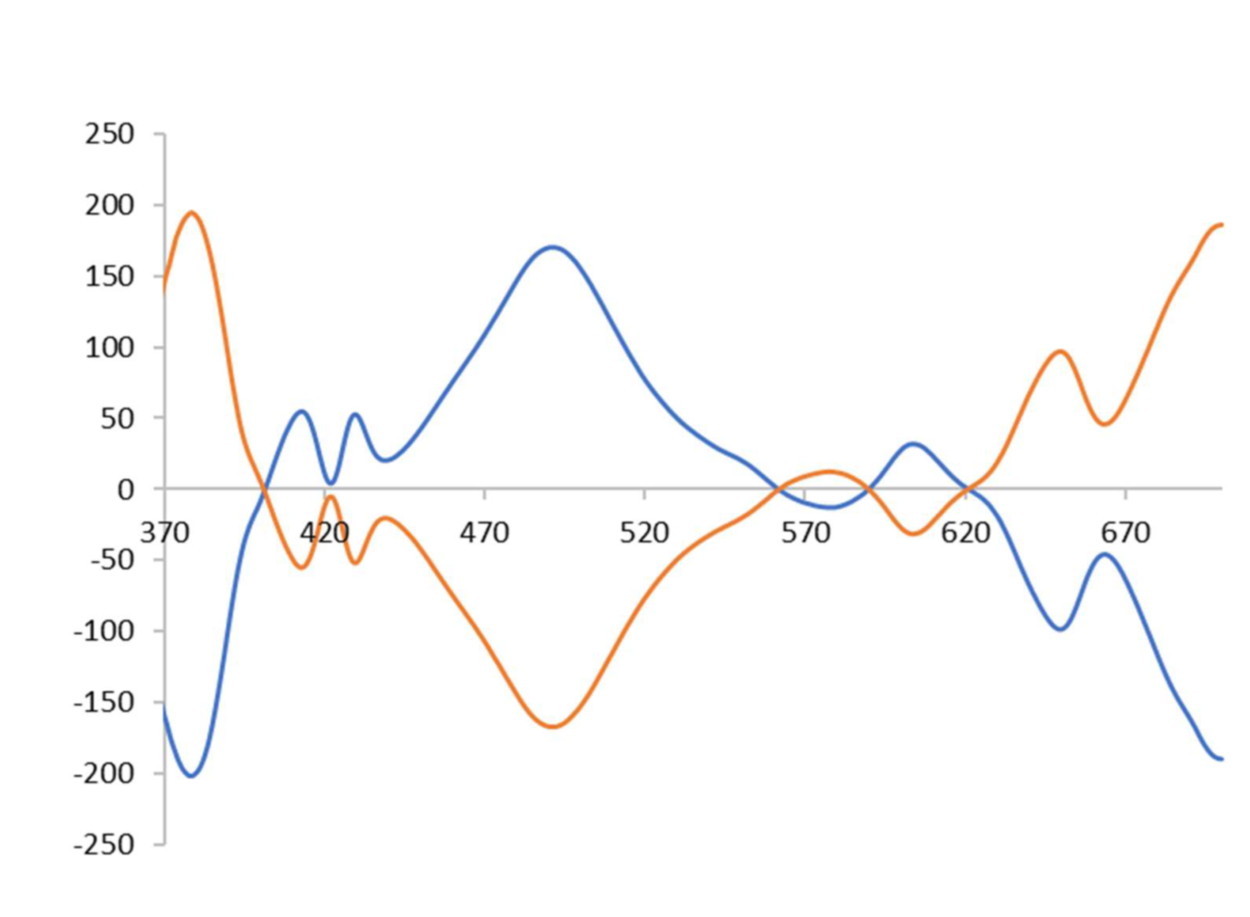
A second application will consist in the development of chiral visible light chromophores as suitable materials for sensing circularly polarized light (CPL). We will adapt the H1 prototype for the binding of fullerenes, and we will study the transfer of chirality from the host to achiral commercially available fullerenes by studying the resulting complexes by electronic circular dichroism (ECD).
These goals will be achieved by a combined experimental-computational approach that will allow us to identify the molecular basis of the chirality transfer phenomena involved, establishing rational design principles for the development of applications based on the relay of chirality through non-covalent interactions.